Recently, in the U.S., there has been a shortage of an anti-anxiety drug, buspirone. The American Society of Health-System Pharmacists (ASHP) lists shortages, and as of Jan. 31, 2019, noted shortages of buspirone tablets manufactured by Accord Healthcare, Mylan and Teva Pharmaceutical, and that the companies did not provide a reason for the shortage.
Recently, in the U.S., there has been a shortage of an anti-anxiety drug, buspirone. The American Society of Health-System Pharmacists (ASHP) lists shortages, and as of Jan. 31, 2019, noted shortages of buspirone tablets manufactured by Accord Healthcare, Mylan and Teva Pharmaceutical, and that the companies did not provide a reason for the shortage.
Accord’s were on back order with an estimated release date of late-March 2019. Mylan said theirs were also on back order and estimated a release date of late-January to early-March, although for some dosages they had no estimated release date. Teva also suggested release dates of early- to mid-February or early-March for some dosages.
Buspirone is the only anti-anxiety medication of its kind.
Although buspirone is not a commonly used drug, “It occupies a special place in psychopharmacology because of its unique nature,” James W. Murrough, M.D., Ph.D., director of the Mood and Anxiety Disorders Program and associate professor of psychiatry and neuroscience at the Icahn School of Medicine at Mount Sinai, tells SELF. “It’s a very important piece of what we can offer patients who have anxiety.”
How does Buspirone (BuSpar) effect serotonin levels?
Buspirone [BuSpar] is an anti-anxiety medication unrelated to most typical “sedatives”, such as Valium, Xanax, etc. Buspirone is effective for so-called generalized or “free-floating” anxiety, but not for panic attacks. Buspirone works via the brain chemical (neurotransmitter) serotonin. While we don’t know exactly what causes anxiety on a chemical level, there is evidence that either too much or too little serotonin is involved–the brain preferring a serotonin level that is just right.
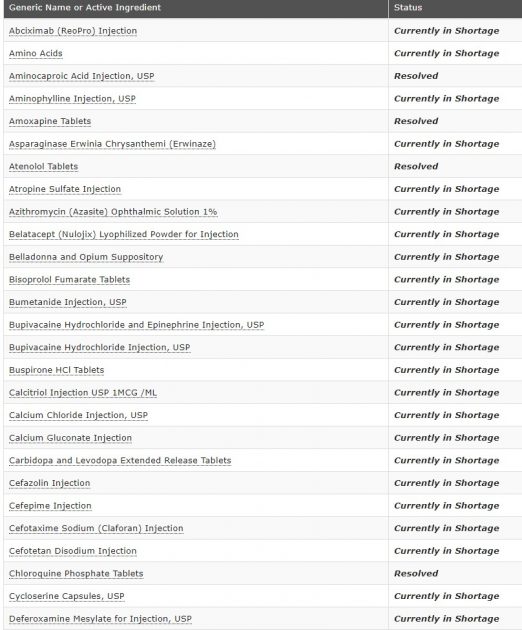
FDA Drug Shortages
A drug receives Resolved status when the Drug Shortages Staff (DSS) determines that the market is covered, based on information from all manufacturers. The market is considered covered when supply is available from at least one manufacturer to cover total market demand. However, some manufacturers may not have all presentations available. DSS monitors the supply of products with Resolved status. For the most current supply information, contact the manufacturers.